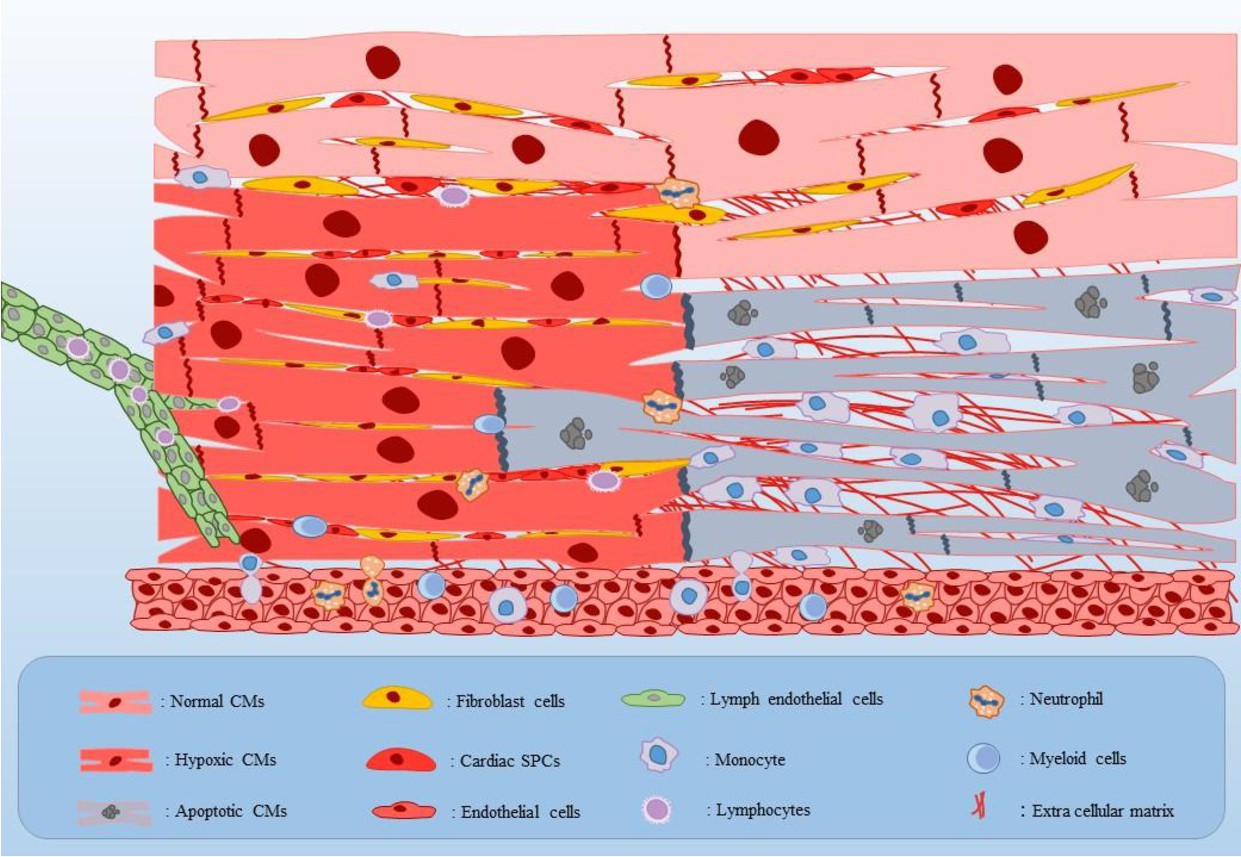
Fig. 2. Schematic representation of the AMI inflammatory microenvironment. Different populations of the fibroblasts, endogenic SPCs, and infiltrated immune cells into the separated parts of normal, hypoxic, and dead myocardium have been shown. Initially after hypoxia, injured CMs, fibroblasts, and endothelial cells actively secrete some of the major pro-inflammatory cytokines such as TNF-α, IL-1β, and also IL-6 into the hypoxic areas. In addition, endogenous SPCs proliferation would be amplified in response to the hypoxia. Next, expression of some important integral membrane proteins including CXC and CC chemokines as well as up-regulation of adhesion molecules on the endothelial cells cause infiltration of inflammatory leucocytes including monocytes, neutrophils, and myeloid cells from the vessels and lymphocytes from the lymph into the infarcted areas. Afterwards, in addition to facilitating the healing process of an injured heart through removing the debris using phagocytosis and secreting some growth factors, recruited immune cells create a stress full inflammatory condition into the infarcted heart tissue via secreting pro-inflammatory cytokines. Abbreviations: CMs: cardiomyocytes, and SPCs: stem/progenitor cells.